In-vitro testing technician
Other denominations
Laboratory assistant; Laboratory technician
Description
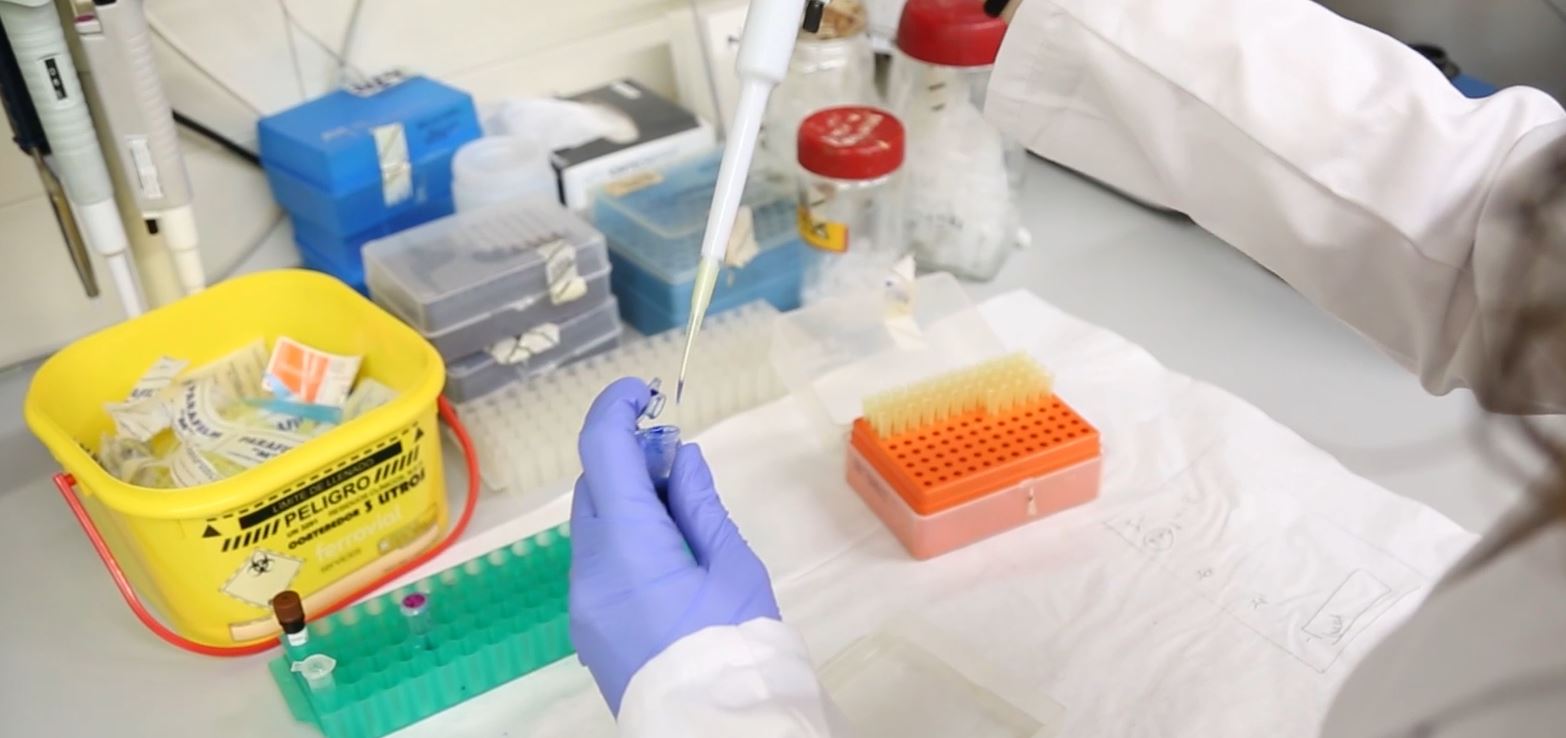
The purpose of in vitro techniques is to describe the effects of an experimental variable introduced for a particular product (biological, pharmaceutical, cosmetics, etc.) in a subset of the constitutional components of an organism such as organs, tissues, cells, cellular components, proteins and/or receptors, which can be carried out in test tubes, petri dishes or wells to carry out high-performance, automated assays (HTS - High Throughput Screening). The in-vitro testing technician is the person in charge of carrying out this kind of assay to assess pharmacological or therapeutic effects, establish the action mechanisms involved, potential toxicity, and means of distribution and excretion (ADME-T studies: absorption-distribution-metabolism-excretion and toxicity) of new drugs or cosmetics. These tests reduce the time and cost involved in the study of new product and the number of animal studies (although these are not a substitute, seeing as animal studies are required by law in order to guarantee safety in patients). Any company wishing to initiate the costly process of developing a new product therefore needs data to support this decision and allow the maximisation of investments.
Tasks
- Conduct experiments according to the protocol and study design established with the main research scientist or head of department.
- Prepare the materials and equipment required to carry out the various necessary tests and daily calibrations.
- Obtain experimental results, to be discussed and reviewed with the person in charge.
- In the case of cell culture laboratories, he/she must ensure that the intrinsic quality of cell lines is maintained and cell growth rates controlled.
- Stay informed as to the appearance of new technologies.
- Suggest improvements for standard testing protocols.
- Must often work under special conditions such as Good Laboratory Practive (GLP) or other pharmaceutical standards.
- Draw up the Standard Operating Procedures (SOP) required to work in GLP conditions under the supervision of the person in charge of the department and according to criteria established by the quality assurance department.










 | Catalan | Beginner
| Catalan | Beginner | Catalan | Advanced
| Catalan | Advanced
 Open
Open | English | Beginner
| English | Beginner