Pharmaceutical laboratory technician
Other denominations
Bioanalyst; laboratory analyst; laboratory technician or assistant; laboratory research technician, physicochemical analyst.
Description
R&D laboratories undergo constant updates, using new methods and instruments. The analysis of biological fluids and tissues, the quality control of raw materials to produce a drug and the control of excipients and formulation are essential with regard to obtaining information on the effects of the product on the organism, the physical-chemical properties of the raw material being developed and its suitability in terms of environmental and safety requirements.
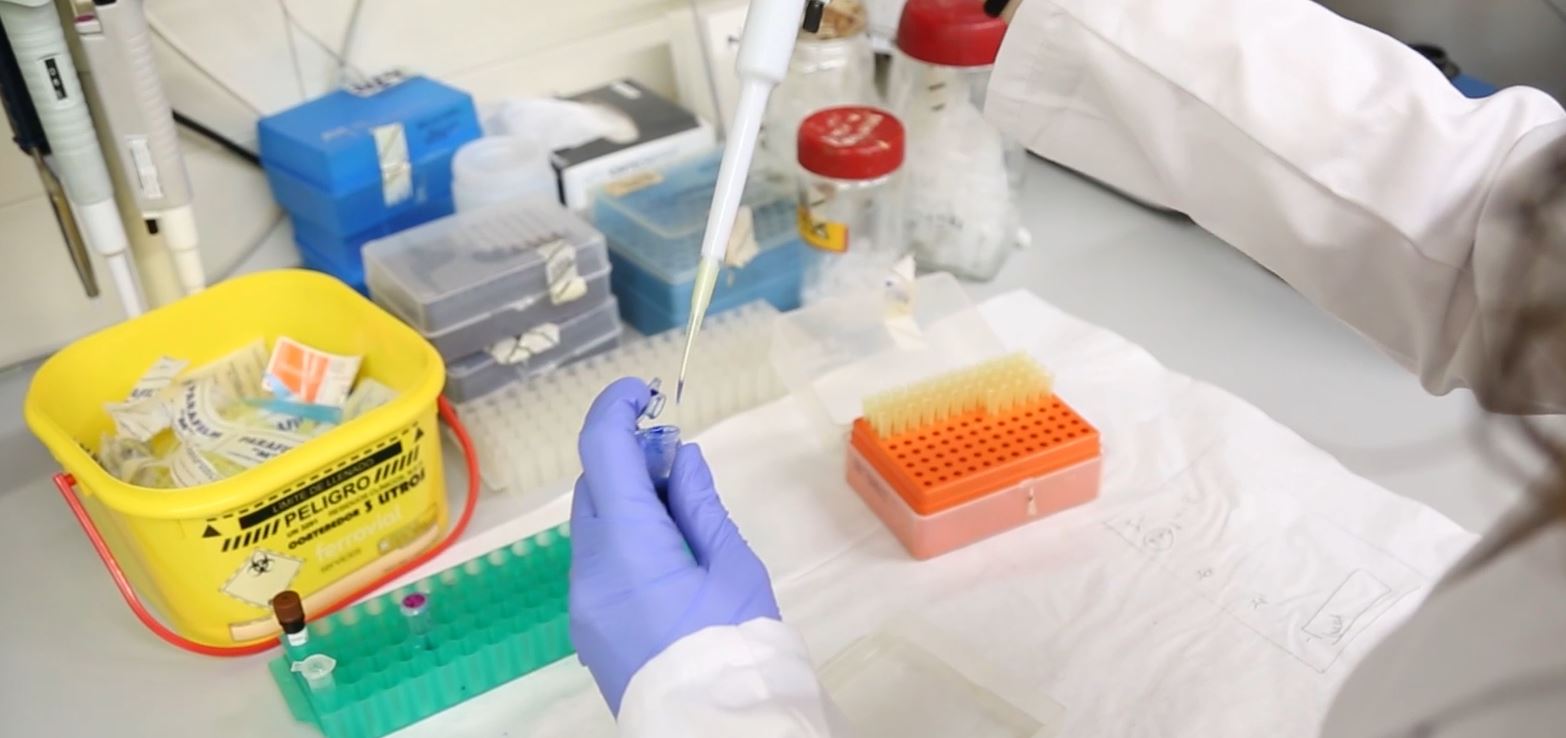
The pharmaceutical laboratory technician is a laboratory assistant who is in charge of analysing raw materials or biological origin such as cells, biological tissues or fluids, new chemical or biological entities or raw materials or finished products, depending on the scientific department s/he operates in (pharmacokinetics, medical or galenic chemistry, respectively).
Tasks
- With regard to medical and galenic chemistry, this person carries out an instrumental analysis of synthesized and formulated products using chromatography and identification techniques such as HPLC/UV and LC/MS, to name the ones most commonly used.
- With regard to biological samples, this person defines and prepares the assay techniques used either for new products or to update existing ones. Assay techniques used include spectroscopy, immunoelectrophoresis, RIA (radioinmunoassay), ELISA (enzyme-linked immunoabsorbent assay) and DNA sequencing, depending on the indications of the supplier company and on the basis of the experimental procedure devised in partnership with the main research scientist in charge.
- Draw up reports detailing any potential anomalies identified in the analysis.
- Draw up internal equipment calibration maintenance reports.
- Draw up sections of the speciality record (analitycal methods, validations, reference templates and compounds and finished product stability).
- Participate in the implementation of new techniques and procedures.
- In the case of studies carried out under low quality standards (Good Laboratory Practice,Good Clinical Clinical or Good Production Practice), follow procedures, inform of any incidents and/or deviations and enter all of the data obtained in approved systems.
- May draw up Standard Operating Procedures (SOP) for techniques under his/her authority.
- Receive audits and quality control reports.










 | Catalan | Beginner
| Catalan | Beginner | Catalan | Advanced
| Catalan | Advanced
 Open
Open | English | Beginner
| English | Beginner